CORD BLOOD
Product Overview
-

Collection Methods
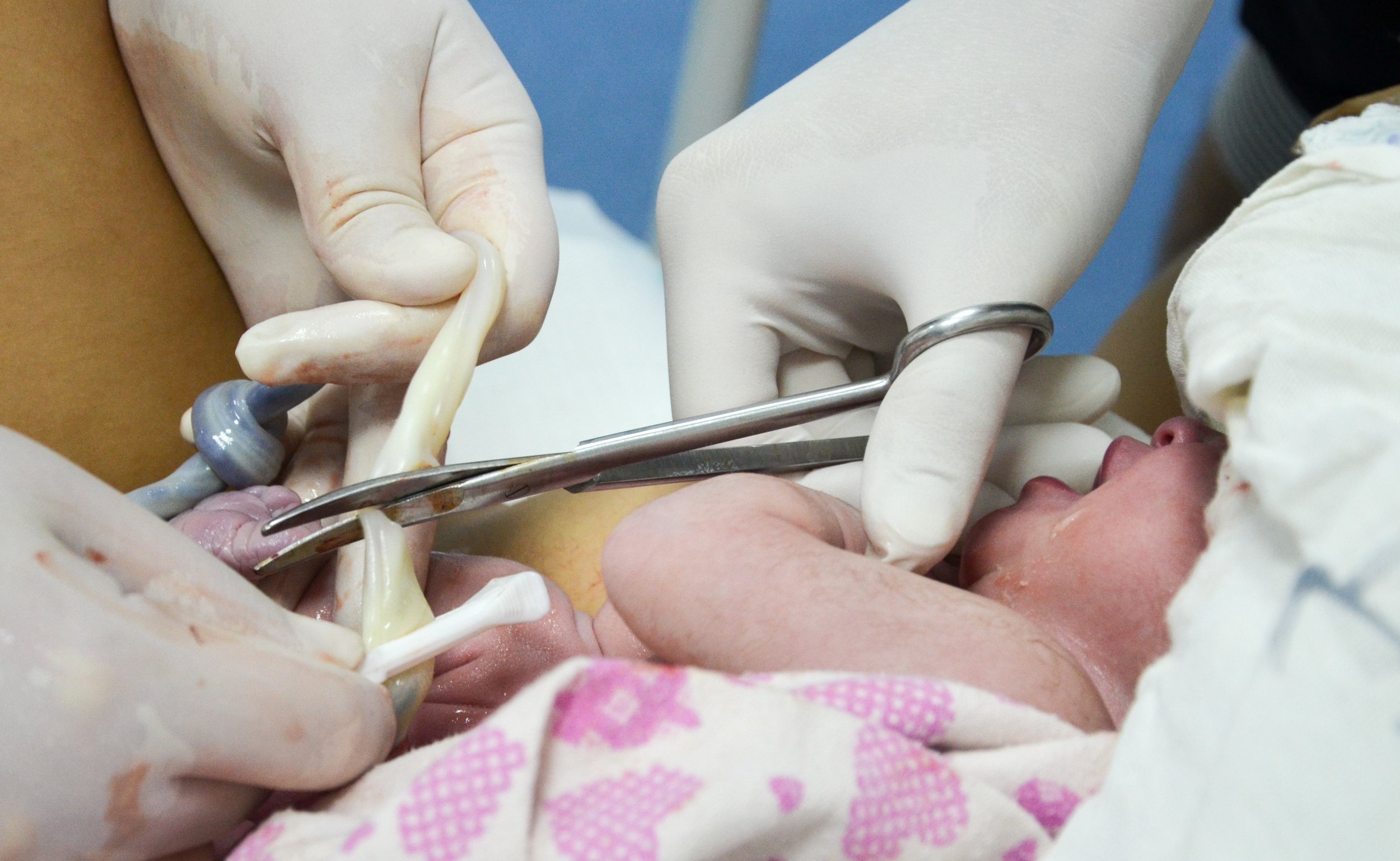
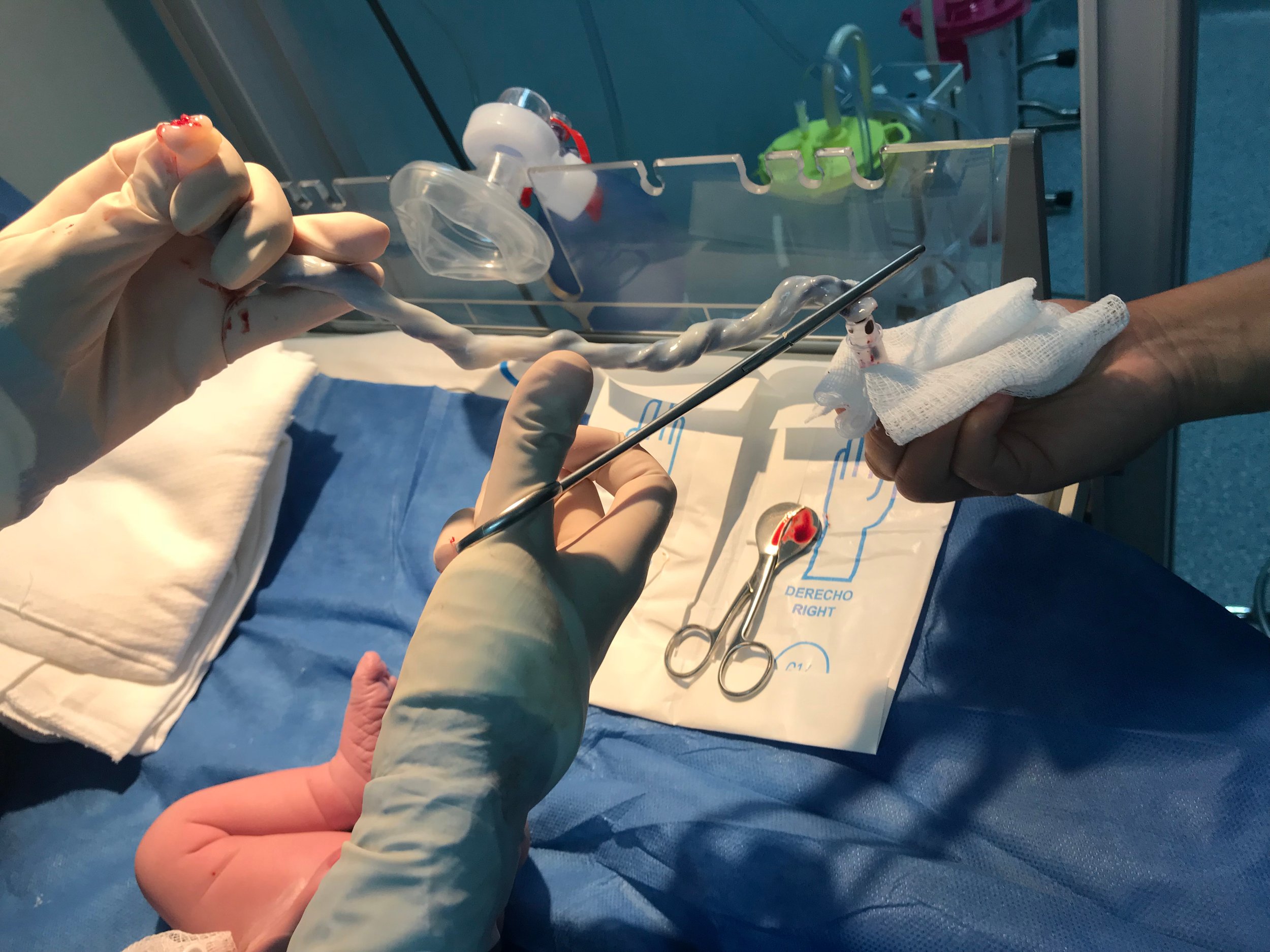
Post placenta delivery, cord blood is collected from the umbilical cord using aseptic technique. The cord blood is collected into a sterile 210mL PVC collection kit. Ideally, >120mL is collected, but due to biological factors some variability occurs.
-
Eligibility Determination
Donors are screened according to 21CFR 1271.75 using the questions found on the AABB uniform donor history questionnaire for cell therapy donors.
Additional questions can be added to a supplemental screening tool upon request.
-
Infectious Disease Testing
Testing is performed for all relevant communicable disease agents defined by the current edition of 21 CFR 610.40 and 21 CFR 1271.75.
All testing is done in compliance with 21 CFR 1271.80 and 21 CFR 1271.85. Only reagents that have been FDA cleared for donor screening are used. Results of testing on the screening date and the date of collection can be provided on the COA if requested.
-
A standard IRB approved consent is used to inform the donor of the risk and reward of the donation. The donor is fully aware that they can discontinue participation at anytime during the process.
-
Testing is performed for all relevant communicable disease agents defined by the current edition of 21 CFR 610.40 and 21 CFR 1271.75.
All testing is done in compliance with 21 CFR 1271.80 and 21 CFR 1271.85. Only reagents that have been FDA cleared for donor screening are used. Results of testing on the screening date and the date of collection can be provided on the COA if requested.
-
The cord blood collection kit used contains 35mL of Citrate Phosphate Dextrose (CPD) anticoagulant solution.
-
Sterility testing is performed on all units. The test method uses the Biomerieux BACT/ALERT system that complies with <USP> 71. Cultures are incubated for 14 days.
-
The 7AAD assay can be added to the flow cytometry enumeration marker to access the viability of specific cell populations.
-
A certificate of Analysis (COA) is provided with each product. The standard COA will include donor demographics and relative cell counts. The content of the COA can be customized to meet specific client needs including IDT and screening assays performed on the date of screening and the date of collection.
-
All donor identifying information is confidential. While donor and product assessment data can be provided to a client, the identity of the donor is never provided. Non-Disclosure Agreements are initiated with all clients and their product and process information is not shared with any other entity or individual.
-
Quality systems have been established and include a quality plan, identification of critical control points (CCP), and key elements that provide process control for each CCP. Supplies, reagents, and equipment are verified against vendor claims and processes are validated using written IQ, OQ and PQ validation plans. SOPs are in place for each process step and all relevant data is documented concurrent with the performance of the task. Training programs for staff include documented assessments of understanding. Each element of 21CFR 211 and 21CFR 1271 are included in the quality system.
-
Using the Sepax cool mix system, a cold DMSO solution is slow injected into the cooling cord blood unit.
Once the cryopreservation agent is added, controlled rate freezing protocols are used to freeze the unit.
-
Using the Sepax-Cytiva system, red blood cells and plasma are removed from the cord blood unit. This occurs in an automated closed system.


